Sunscreens contain one or more of the following ingredients:
- Organic chemical compounds that absorb ultraviolet light.
- Inorganic particulates that reflect, scatter, and absorb UV light (such as titanium dioxide, zinc oxide, or a combination of both).
- Organic particulates that mostly absorb light like organic chemical compounds, but contain multiple chromophores, may reflect and scatter a fraction of light like inorganic particulates, and behave differently in formulations than organic chemical compounds. An example is Tinosorb M. Since the UV-attenuating efficacy depends strongly on particle size, the material is micronised to particle sizes below 200 nm. The mode of action of this photostable filter system is governed to about 90% by absorption and 10% by scattering of UV light.
Medical organizations such as the American Cancer Society recommend the use of sunscreen because it prevents the squamous cell carcinoma and the basal cell carcinoma.[3] However, the use of sunscreens is controversial for various reasons. Many sunscreens do not block UVA radiation, which does not cause sunburn but can increase the rate of melanoma, another kind of skin cancer, so people using sunscreens may be exposed to a high level UVA without realizing it.
It is often colloquially called sun tan lotion due to the product's similar name, although sun tan lotion is used to absorb UV rays rather than block them.
Sun protection factor (SPF)

Two photographs showing the effect of applying sunscreen in visible light and in UVA. The photograph on the right was taken using ultraviolet photography shortly after application of sunscreen to half of the face.
The SPF is the amount of UV radiation required to cause sunburn on skin with the sunscreen on, relative to the amount required without the sunscreen.[9] There is a popular misconception that SPF relates to time of solar exposure. For example, many consumers believe that, if they normally get sunburn in one hour, then an SPF 15 sunscreen allows them to stay in the sun 15 hours (i.e., 15 times longer) without getting sunburn. This is not true because SPF is not directly related to time of solar exposure but to amount of solar exposure. Although solar energy amount is related to solar exposure time, there are other factors that impact the amount of solar energy, like the time of day. This is because, during early morning and late afternoon, the sun's radiation must pass through more of the Earth's atmosphere before it gets to you. In practice, the protection from a particular sunscreen depends on factors such as:
- The skin type of the user.
- The amount applied and frequency of re-application.
- Activities in which one engages (for example, swimming leads to a loss of sunscreen from the skin).
- Amount of sunscreen the skin has absorbed.
Owing to consumer confusion over the real degree and duration of protection offered, labeling restrictions are in force in several countries. In the EU sunscreen labels can only go up to SPF 50+ (actually indicating a SPF of 60 or higher)[16] while Australia's upper limit is 30+.[17] The United States does not have mandatory, comprehensive sunscreen standards, although a draft rule has been under development since 1978. In the 2007 draft rule, Food and Drug Administration (FDA) proposed to institute the labelling of SPF 50+ for sunscreens offering more protection. This and other measures were proposed to limit unrealistic claims about the level of protection offered (such as "all day protection").[18]
Mathematically, the SPF is calculated from measured data as
The above means that the SPF is not simply the inverse of the transmittance in the UV-B region. If that were true, then applying two layers of SPF 5 sunscreen would be equivalent to SPF 25 (5 times 5). The actual combined SPF is always lower than the square of the single-layer SPF.
[edit] Measurements of UVA protection
[edit] Persistent pigment darkening (PPD)
The persistent pigment darkening (PPD) method is a method of measuring UVA protection, similar to the SPF method of measuring UVB light protection. Originally developed in Japan, it is the preferred method used by manufacturers such as L'Oreal.Instead of measuring erythema or reddening of the skin, the PPD method uses UVA radiation to cause a persistent darkening or tanning of the skin. Theoretically, a sunscreen with a PPD rating of 10 should allow a person 10 times as much UVA exposure as would be without protection. The PPD method is an in vivo test like SPF. In addition, Colipa has introduced a method which, it is claimed, can measure this in vitro and provide parity with the PPD method.[21]

The UVA seal used in the EU
[edit] Star rating system
In the UK and Ireland, the Boots star rating system is a proprietary in vitro method used to describe the ratio of UVA to UVB protection offered by sunscreen creams and sprays. Based on original work by Prof. Brian Diffey at Newcastle University, the Boots Company in Nottingham, UK, developed a standard method which has been adopted by most companies marketing these products in the UK. The logo and methodology of the test are licenced for a token fee to any manufacturer or brand of sunscreens that are sold in the Boots retail chain, provided the products to which the logo is applied perform to the standard claimed. Own Label products exclusively sold in other retailers are now excluded from the terms of the licence. It should not be confused with SPF, which is measured with reference to burning and UVB. One-star products provide the least ratio of UVA protection; five-star products are best. The method has recently been revised in the light of the Colipa UVA PF test, and with the new EU recommendations regarding UVA PF. The method still uses a spectrophotometer to measure absorption of UVA vs UVB; the difference stems from a requirement to pre-irradiate samples (where this was not previously required) to give a better indication of UVA protection, and of photostability when the product is used. With the current methodology, the lowest rating is three stars, the highest being five stars.In August 2007, the FDA put out for consultation the proposal that a version of this protocol be used to inform users of American product of the protection that it gives against UVA [23]
[edit] Differences between sunblock and sunscreen
Although it is a common misconception[citation needed] that sunblock and sunscreen are both the same, they are not. They have similar properties and are both important in caring of the skin, sunblock is opaque and is stronger than sunscreen since it is able to block a majority of the UVA/UVB rays and radiation from the sun, thus not having to be reapplied several times a day. Titanium dioxide and zinc oxide are two of the important ingredients in sunblock.Sunscreen is more transparent once applied to the skin and also has the ability to protect against UVA/UVB rays as well, although the sunscreen's ingredients have the ability to break down at a faster rate once exposed to sunlight, and some of the radiation is able to penetrate to the skin. In order for sunscreen to be more effective the user needs to consistently reapply at least every two hours, and use a higher SPF.[24]
However, that distinction is mostly used for marketing, and the FDA has in fact considered banning the term "sunblock" from marketing claims as it considers it misleading.[25]
For total protection against damage from the sun, the skin needs to be protected from UVA, UVB and IRA (Infra Red Energy). Roughly 35% of solar energy is IRA.[26]
[edit] Potential health risks
Main article: Sunscreen controversy
As a defense against UV radiation, the amount of the brown pigment melanin in the skin increases when exposed to moderate (depending on skin type) levels of radiation; this is commonly known as a sun tan. The purpose of melanin is to absorb UV radiation and dissipate the energy as harmless heat, blocking the UV from damaging skin tissue. UVA gives a quick tan that lasts for days by oxidizing melanin that was already present and triggers the release of the melanin from melanocytes. UVB on the other hand yields a tan that takes roughly two days to develop because it stimulates the body to produce more melanin. The photochemical properties of melanin make it an excellent photoprotectant.Sunscreen chemicals on the other hand cannot dissipate the energy of the excited state as efficiently as melanin and therefore the penetration of sunscreen ingredients into the lower layers of the skin increases the amount of free radicals and reactive oxygen species (ROS).[6]
Some sunscreen lotions now include compounds such as titanium dioxide which helps protect against UVB rays. Other UVA blocking compounds found in sunscreen include zinc oxide and avobenzone. There are also naturally occurring compounds found in rainforest plants that have been known to protect the skin from UV radiation damage, such as the fern Phlebodium aureum.
Some sunscreen chemicals produce potentially harmful substances if they are illuminated while in contact with living cells.[27][28][29] The amount of sunscreen which penetrates through the stratum corneum may or may not be large enough to cause damage. In one study of sunscreens, the authors write:
The question whether UV filters acts on or in the skin has so far not been fully answered. Despite the fact that an answer would be a key to improve formulations of sun protection products, many publications carefully avoid addressing this question.[30]In an experiment by Hanson et al. that was published in 2006, the amount of harmful reactive oxygen species was measured in untreated and in sunscreen-treated skin. In the first 20 minutes the film of sunscreen had a protective effect and the number of ROS species was smaller. After 60 minutes, however, the amount of absorbed sunscreen was so high that the amount of ROS was higher in the sunscreen-treated skin than in the untreated skin.[6]
George Zachariadis and E Sahanidou of the Laboratory of Analytical Chemistry, at Aristotle University, in Thessaloniki, Greece, have now carried out an ICP-AES analysis of several commercially available sunscreen creams and lotions. "The objective was the simultaneous determination of titanium and several minor, trace or toxic elements (aluminum, zinc, magnesium, iron, manganese, copper, chromium, lead, and bismuth) in the final products," the researchers say. They concluded that "Most of the commercial preparations that were studied showed generally good agreement to the ingredients listed on the product label." However, they also point out that the quantitative composition of the products tested cannot be assessed because the product labels usually do not provide a detailed break down of all ingredients and their concentrations. They also point out that, worryingly, their tests consistently revealed the presence of elements not cited in the product formulation, which emphasized the need for a standardized and official testing method for multi-element quality control of these products.[31]
Some epidemiological studies indicate an increased risk of malignant melanoma for the sunscreen user.[32][33][34][35][36][37][38][39] Despite these studies, no medical association has published recommendations to not use sunblock. Different meta-analysis publications have concluded that the evidence is not yet sufficient to claim a positive correlation between sunscreen use and malignant melanoma.[40][41]
Adverse health effects may be associated with some synthetic compounds in sunscreens.[42] In 2007 two studies by the CDC highlighted concerns about the sunscreen chemical oxybenzone (benzophenone-3). The first detected the chemicals in greater than 95% of 2000 Americans tested, while the second found that mothers with high levels of oxybenzone in their bodies were more likely to give birth to underweight baby girls.[43]
The use of sunscreen also interferes with vitamin D production, leading to deficiency in Australia after a government campaign to increase sunscreen use.[44] Doctors recommend spending small amounts of time in the sun without sun protection to ensure adequate production of vitamin D.[45] When the UV index is greater than 3 (which occurs daily within the tropics and daily during the spring and summer seasons in temperate regions) adequate amounts of vitamin D3 can be made in the skin after only ten to fifteen minutes of sun exposure at least two times per week to the face, arms, hands, or back without sunscreen. With longer exposure to UVB rays, an equilibrium is achieved in the skin, and the vitamin simply degrades as fast as it is generated.[46]
Concerns have been raised regarding the use of nanoparticles in sunscreen.[47] Theoretically, sunscreen nanoparticles could increase rates of certain cancers, or diseases similar to those caused by asbestos.[48] In 2006 the Therapeutic Goods Administration of Australia concluded a study and found:
"There is evidence from isolated cell experiments that zinc oxide and titanium dioxide can induce free radical formation in the presence of light and that this may damage these cells (photo-mutagenicity with zinc oxide). However, this would only be of concern in people using sunscreens if the zinc oxide and titanium dioxide penetrated into viable skin cells. The weight of current evidence is that they remain on the surface of the skin and in the outer dead layer (stratum corneum) of the skin." [47]
[edit] Active ingredients
The principal ingredients in sunscreens are usually aromatic molecules conjugated with carbonyl groups. This general structure allows the molecule to absorb high-energy ultraviolet rays and release the energy as lower-energy rays, thereby preventing the skin-damaging ultraviolet rays from reaching the skin. So, upon exposure to UV light, most of the ingredients (with the notable exception of avobenzone) do not undergo significant chemical change, allowing these ingredients to retain the UV-absorbing potency without significant photodegradation.[4] A chemical stabilizer is included in some sunscreens containing avobenzone to slow its breakdown - examples include formulations containing Helioplex[49] and AvoTriplex.[50] The stability of avobenzone can also be improved by bemotrizinol,[51] octocrylene[52] and various other photostabilisers.[edit] FDA allowable ingredients
The following are the FDA allowable active ingredients in sunscreens:| UV-filter | Other names | Maximum concentration | Permitted in these countries | Results of safety testing |
|---|---|---|---|---|
| p-Aminobenzoic acid | PABA | 15% (5% EC-will be banned from sale to consumers from 8 October 2009) | EC, USA, AUS | Protects against skin tumors in mice.[53][54][55] Shown to increase DNA defects, however, and is now less commonly used. |
| Padimate O | OD-PABA, octyldimethyl-PABA, σ-PABA | 8% (EC,USA,AUS) 10% (JP) (Not currently supported in EU and may be delisted) | EC, USA, AUS, JP | Not tested |
| Phenylbenzimidazole sulfonic acid | Ensulizole, Eusolex 232, PBSA, Parsol HS | 4% (US,AUS) 8% (EC) 3% (JP) | EC,USA, AUS, JP | Genotoxic in bacteria[56] |
| Cinoxate | 2-Ethoxyethyl p-methoxycinnamate | 3% (US) 6% (AUS) | USA, AUS | Not tested |
| Dioxybenzone | Benzophenone-8 | 3% | USA, AUS | Not tested |
| Oxybenzone | Benzophenone-3, Eusolex 4360, Escalol 567 | 6% (US) 10% (AUS,EU) 5% (JP) | EC, USA, AUS, JP | Not tested |
| Homosalate | Homomethyl salicylate, HMS | 10% (EC, JP) 15% (US,AUS) | EC, USA, AUS, JP | Not tested |
| Menthyl anthranilate | Meradimate | 5% | USA, AUS | Not tested |
| Octocrylene | Eusolex OCR, 2-cyano-3,3diphenyl acrylic acid, 2-ethylhexylester | 10% | EC,USA, AUS, JP | Increases ROS[6] |
| Octyl methoxycinnamate | Octinoxate, EMC, OMC, Ethylmethoxycinnamate, Escalol 557, 2-ethylhexyl-paramethoxycinnamate, Parsol MCX | 7.5% (US) 10% (EC,AUS)20% (JP) | EC,USA, AUS, JP | |
| Octyl salicylate | Octisalate, 2-Ethylhexyl salicylate, Escalol 587, | 5% (EC,USA,AUS) 10% (JP) | EC,USA, AUS, JP | Not tested |
| Sulisobenzone | 2-Hydroxy-4-Methoxybenzophenone-5-sulfonic acid, 3-benzoyl-4-hydroxy-6-methoxybenzenesulfonic acid, Benzophenone-4, Escalol 577 | 5% (EC) 10% (US, AUS, JP) | EC,USA, AUS, JP | |
| Trolamine salicylate | Triethanolamine salicylate | 12% | USA, AUS | Not tested |
| Avobenzone | 1-(4-methoxyphenyl)-3-(4-tert-butyl phenyl)propane-1,3-dione, Butyl methoxy dibenzoylmethane, BMDBM, Parsol 1789, Eusolex 9020 | 3% (US) 5% (EC,AUS)10% (JP) | EC, USA, AUS, JP | Not available[57] |
| Ecamsule | Mexoryl SX, Terephthalylidene Dicamphor Sulfonic Acid | 10% | EC,AUS (US:Approved in certain formulations up to 3% via New Drug Application (NDA) Route) | Protects against skin tumors in mice[58][59][60] |
| Titanium dioxide | CI77891 | 25% (No limit Japan) | EC,USA, AUS, JP | Not tested |
| Zinc oxide | 25% (US) 20% (AUS) (EC-25% provided particle size >100 nm) (Japan, No Limit) | EC,USA, AUS, JP | Protects against skin tumors in mice[58] |
| UV-filter | Other names | Maximum concentration | Permitted in |
|---|---|---|---|
| 4-Methylbenzylidene camphor | Enzacamene, Parsol 5000, Eusolex 6300, MBC | 4%* | EC, AUS |
| Tinosorb M | Bisoctrizole, Methylene Bis-Benzotriazolyl Tetramethylbutylphenol, MBBT | 10%* | EC, AUS, JP |
| Tinosorb S | Bis-ethylhexyloxyphenol methoxyphenol triazine, Bemotrizinol, BEMT, anisotriazine | 10% (EC, AUS) 3% (JP)* | EC, AUS, JP |
| Neo Heliopan AP | Bisdisulizole Disodium, Disodium phenyl dibenzimidazole tetrasulfonate, bisimidazylate, DPDT | 10% | EC, AUS |
| Mexoryl XL | Drometrizole Trisiloxane | 15% | EC, AUS |
| Benzophenone-9 | Uvinul DS 49, CAS 3121-60-6, Sodium Dihydroxy Dimethoxy Disulfobenzophenone [63] | 10% | JP |
| Uvinul T 150 | Octyl triazone, ethylhexyl triazone, EHT | 5% (EC, AUS) 3% (JP)* | EC, AUS |
| Uvinul A Plus | Diethylamino Hydroxybenzoyl Hexyl Benzoate | 10% (EC,JP) | EC , JP |
| Uvasorb HEB | Iscotrizinol, Diethylhexyl butamido triazone, DBT | 10% (EC) 5% (JP) * | EC, JP |
| Parsol SLX | Dimethico-diethylbenzalmalonate, Polysilicone-15 | 10% | EC, AUS, JP |
| Isopentenyl-4-methoxycinnamate | Isoamyl p-Methoxycinnamate, IMC, Neo Heliopan E1000, Amiloxate | 10% * | EC, AUS |
Sunscreen Cream o/w
| Formula Standart | Formula Pembanding | Formula Modifikasi | Fungsi Bahan | |
| Harry’s Cosmetic p.253 | | UV A 1% | | |
| UV B 3% | | |||
| Asam Stearat 3,5% | HPE5’th P.738 | |||
| Cetyl Alkohol 2% | HPE 5’th P.156 | |||
| TEA 1,0% | | |||
| TEA lauryl sulfat 0,75% | | |||
| Nipagin 0,18% | HPE 5’th P.466 | |||
| Nipasol 0,02% | HPE 5’th P.629 | |||
| Propilon Glikol 2% | HPE 5’th P.624 | |||
| Water 80,55% | | |||
Alasan: Giv-tan F hanya sebagai UV B à sehingga ditambah UV A



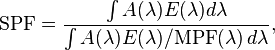

Tidak ada komentar:
Posting Komentar